0115-1911 : Rimantadine Hydrochloride 100 mg Oral Tablet
| NDC: | 0115-1911 |
| Labeler: | Global Pharmaceuticals, Division of Impax Laboratories Inc. |
| Product Type: | Human Prescription Drug |
| Drug Name: |  Rimantadine Hydrochloride Rimantadine Hydrochloride |
| Dosage Form: | Oral Tablet, Film Coated |
| Application #: | ANDA076132 |
| Rev. Date: |
Appearance:
| Markings: | G;1911 |
| Shapes: |
Oval |
| Colors: |
 Orange Orange |
| Size (mm): | 11 |
| Segments: * | 1 |
* Segments = the number of equally sized pieces which the pill can be broken into. In this case, a value of 1 indicates a solid pill with no score lines. | |
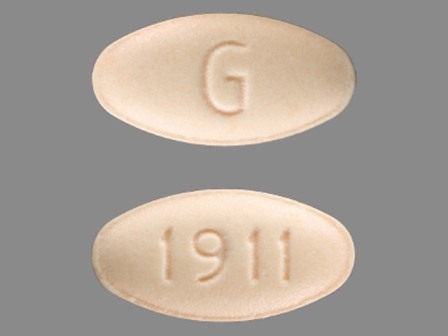
The above image is provided by the U.S. National Library of Medicine (NLM) and is not part of the official label.
NDC Package Codes:
- 0115-1911-01: 100 TABLET, FILM COATED IN 1 BOTTLE (0115‑1911‑01)
- 0115-1911-02: 500 TABLET, FILM COATED IN 1 BOTTLE (0115‑1911‑02)
Active Ingredients:
- Rimantadine Hydrochloride
Dosage Strength:
- 100 mg
Inactive Ingredients:
- Hypromellose
- Magnesium Stearate
- Cellulose, Microcrystalline
- Sodium Starch Glycolate Type a Potato
- Fd&c Yellow No. 6
- Polyethylene Glycol
Pharmaceutical Classes:
- Influenza A M2 Protein Inhibitor [EPC]
- M2 Protein Inhibitors [MoA]
Related Products:
Based on records with the same trade name.- 16590-202 Rimantadine Hydrochloride 100 mg Oral Tablet by Stat Rx USA LLC
- 50090-1775 Rimantadine Hydrochloride 100 mg Oral Tablet, Film Coated by A-s Medication Solutions
- 52959-305 Rimantadine Hydrochloride 100 mg Oral Tablet by H.j. Harkins Company, Inc.
- 63629-3552 Rimantadine Hydrochloride 100 mg Oral Tablet, Film Coated by Bryant Ranch Prepack
- 68151-2103 Rimantadine Hydrochloride 100 mg Oral Tablet, Film Coated by Carilion Materials Management
NDC QR Code
Scan the QR code below to easily reference this data in the future:
< Prev: 0115-1864Next: 0115-2011 >
Note: The RxChat NDC Database uses publicly available data from the FDA and the U.S. National Library of Medicine (NLM); The NLM is not responsible for the data presented and does not endorse or recommend this or any other product. While we make every effort to ensure that the information presented is accurate, you should assume that all results are unvalidated. To report any errors or inconsistencies please contact us.